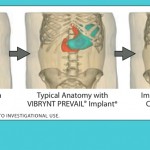
 A new adjustable weight loss device has been approved by the FDA for clinical study in the US. The VIBRYNT PREVAIL Implant System is an investigational device that is designed to assist with weight loss by limiting food consumption. It is implanted on top of the stomach laparoscopically and restricts the stomach’s ability to expand. Sitting in the space that the stomach would normally expand into during a meal, the device allows patients to feel full after eating less food, which encourages weight loss. PREVAIL is filled with a saline solution and can be adjusted over time according to the patient’s needs; in this sense, it is similar to the lap-band which is adjustable. However, the device is intended to function primarily like the gastric sleeve, which involves surgical removal of part of the stomach, leaving a long sleeve or tube-shaped stomach. Unlike the gastric sleeve, however, the PREVAIL System does not alter the gastrointestinal anatomy: there is no cutting, suturing, stapling, nor removal of any part of the stomach. Vibrynt has gone through several phases of research to improve the placement and results with their device.
A new adjustable weight loss device has been approved by the FDA for clinical study in the US. The VIBRYNT PREVAIL Implant System is an investigational device that is designed to assist with weight loss by limiting food consumption. It is implanted on top of the stomach laparoscopically and restricts the stomach’s ability to expand. Sitting in the space that the stomach would normally expand into during a meal, the device allows patients to feel full after eating less food, which encourages weight loss. PREVAIL is filled with a saline solution and can be adjusted over time according to the patient’s needs; in this sense, it is similar to the lap-band which is adjustable. However, the device is intended to function primarily like the gastric sleeve, which involves surgical removal of part of the stomach, leaving a long sleeve or tube-shaped stomach. Unlike the gastric sleeve, however, the PREVAIL System does not alter the gastrointestinal anatomy: there is no cutting, suturing, stapling, nor removal of any part of the stomach. Vibrynt has gone through several phases of research to improve the placement and results with their device.
Many obesity experts are excited about the prospect of another device to treat obesity. PREVAIL was discussed by Dr Jaime Ponce at a session at the Minimally Invasive Surgery Symposium earlier this year, and was introduced by Vibrynt CEO Beverly Huss at the 29th annual meeting of the American Society for Metabolic & Bariatric Surgery (ASMBS) last month. At the ASMBS meeting, Australian researchers reported that in their study of 69 participants with the device, 6 month excess weight loss was 29%, with an average of 12.6% total body weight loss. They also reported that patients with the device demonstrated significant improvements in managing their eating behavior at 6 months.
Dr. Christine Ren Fielding, expert bariatric surgeon in New York who is on PREVAIL’s advisory team, spoke with us about the device. She said, “the PREVAIL system is one of two devices that have been approved by the FDA to conduct scientific trials in the US. It is a novel device which is implanted laparoscopically into the abdominal cavity to cause satiety (the feeling of fullness).” Dr. Ren Fielding added that the early outcomes from Australia are promising in terms of both weight loss and safety.
The device is currently not for sale in the US and is only available through clinical trial. It is designed for people struggling with obesity, with a body mass index (BMI) of 40 or greater, or 35 or greater if the patient has one or more significant obesity-related condition. PREVAIL will be evaluated in an FDA-approved research study called the PREVAIL U.S. Pivotal Study. Vibrynt will soon be announcing the study sites. CEO Huss told us, “We are excited to begin patient enrollment in our US pivotal study and pleased to be working with an esteemed group of bariatric surgeons as our clinical investigators.” In addition to Dr. Ren Fielding, other Doctors of Weight Loss experts – Drs Emma Patterson, Jaime Ponce, and George Fielding – are consultants/advisors for Vibrynt.






Industry & Innovation